This is what a BMJ publication, out today,states in its conclusions: ‘among this group of large clinical trials, non-publication of results was common and the availability of results in the ClinicalTrials.gov database was limited.’
I have highlighted the important bits in bold, as if it isn’t clear to anyone yet, and you may need to read it again, this is a huge problem: the non-publication of results is common and the results of clinical trials remain limited.
I have previously published, on this blog, that a high proportion of clinical trial data is never published, and the evidence backing up this point is is growing.
So what did the authors do this time ?
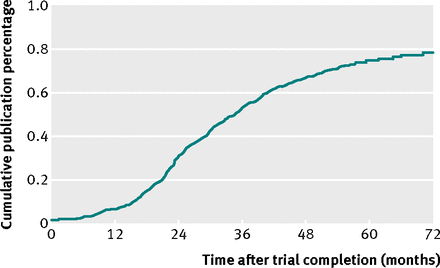
They looked at 585 registered trials, with a sample size larger than 500, of which 29% remained unpublished, after a median of 60months. This is what makes this study slightly different to the others that that have gone before. Smaller trials may have acounted for most of the problem, and by eliminating them we can readily see non-publication is just as bad for those trials with larger samples: those that are most likely to impact on healthcare.
In addtion, industry studies were nearly twice as likely not to be published 32% versus 18% for those rhat received no industry funding. And of the 171 unpublished trials, 78% had no available results in ClinicalTrials.gov.
I am sorry BMJ, but I need to reproduce this slide, as it says it all, and it is too important not to show the reality of FDA regulation; that states, it is mandatory to post summary results in one year (read the paper, it is open access)
At some point, it will become obvious, to those that make all the decisions, that without access to the full evidence-base, we will remain in the dark as to the true effects of any given intervention.
carl
November 2, 2013 at 3:00 pmHere are some news stories related to the BMJ research
Scientists alarmed over ethics of drug trials remaining unpublished up to five years after they’re finished.
http://www.rawstory.com/rs/2013/10/30/scientists-alarmed-over-ethics-of-drug-trials-remaining-unpublished-up-to-five-years-after-theyre-finished/
Big Drug Firms Mobilize Patient Groups to Lobby against Publication of Secret Drug Testing Data
http://www.allgov.com/news/controversies/big-drug-firms-mobilize-patient-groups-to-lobby-against-publication-of-secret-drug-testing-data-130724?news=850649